V-WIPE Zero Full Effective Spectrum
Bossklein V-WIPE Zero alcohol free disinfectant wipes are effective against a wide range of different micro organisms all within a quick contact time of only 60 seconds.
Scroll down to see detailed information about the full efficacy, specific test results and EN standards achieved by the high performance Bossklein V-WIPE Zero disinfectant wipes.
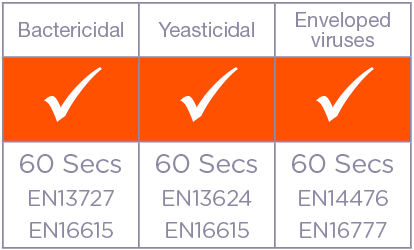
| Bacteria | EN Test | Contact Time | Condition |
| Pseudomonas aeruginosa | EN13727, EN16615 | 60 sec | Dirty/Clean |
| Staphylococcus aureus | EN13727, EN16615 | 60 sec | Dirty/Clean |
| Enterococcus hirae | EN13727, EN16615 | 60 sec | Dirty/Clean |
| Yeast | EN Test | Contact Time | Condition |
| Candida albicans | EN16324, EN16615 | 60 sec | Dirty/Clean |
| Enveloped Virus | EN Test | Contact Time | Condition |
| Vaccinia virus | EN14476, EN16777 | 60 sec | Dirty/Clean |
| Hepatitis B virus (HBV) |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Hepatitis C virus (HCV) |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Hepatitis Delta virus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| HIV |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Influenza virus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Coronavirus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Herpesviridae |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Filoviridae (Ebola) |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Rubella virus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Rabies virus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Measles virus |
EN14476, EN16777 | 60 sec | Dirty/Clean |
| Poxviridae |
EN14476, EN16777 | 60 sec | Dirty/Clean |
The following test methods set out the level of virucidal claim that can be made on a product:
BS EN 14476:2013 + A2:2019 Chemical disinfectants and antiseptics
Quantitative suspension test for the evaluation of virucidal activity in the medical area – Test method and requirements (Phase 2/Step 1)
Surface disinfection products can claim 3 levels of virucidal activity:
1) Virucidal activity 2) Limited spectrum virucidal activity or 3) Virucidal activity against enveloped viruses.
Our alcohol free surface disinfectant products claim ‘Virucidal activity against enveloped viruses’. The standards state testing shall be performed against vaccinia virus. The A2 amendment clarifies that ‘The test for “virucidal activity against enveloped viruses” will cover all enveloped viruses.
No individual tests are required against the above enveloped viruses, as they are covered by the ‘Virucidal activity against enveloped viruses’ claim.
BS EN 16777:2018 Chemical disinfectants and antiseptics
Quantitative nonporous surface test without mechanical action for the evaluation of virucidal activity of chemical disinfectants used in the medical area – Test method and requirements (Phase 2/Step 2)
Other test standards achieved:
BS EN 13727:2012 + A2:2015 Chemical disinfectants and antiseptics
Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of bactericidal activity in the medical area – Test method and requirements (phase 2, step 1).
BS EN 13624:2021 Chemical disinfectants and antiseptics
Quantitative suspension test for the evaluation of fungicidal or yeasticidal activity in the medical area – Test method and requirements (phase 2, step 1).
BS EN 16615:2015 (E) Chemical disinfectants and antiseptics
Chemical disinfectants and antiseptics – Quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4-field test) – Test method and requirements (phase 2, step 2).
Maintaining a sterile clinical environment is critical to ensuring patient safety and preventing cross-contamination. Surface disinfectants play a vital role in infection control protocols by reducing or eliminating microbial contamination on non-porous surfaces. Efficacy claims for these disinfectants are regulated and must be supported by standardised testing to verify their ability to kill or inactivate a broad spectrum of pathogens.
Disinfectants used in dental settings must comply with specific regulatory standards. Key efficacy benchmarks including bactericidal, virucidal, and tuberculocidal activity, must be demonstrated through laboratory testing protocols.
Our products are vigorously tested using European Norms (EN) efficacy test standards. EN tests are a mandatory requirement for biocidal products and disinfectants used on medical devices in Europe and Great Britain. EN standards are recognised globally, and have stricter requirements than other available standards.
