IDactiv Instrument Disinfectant and Cleaner
Product Highlights
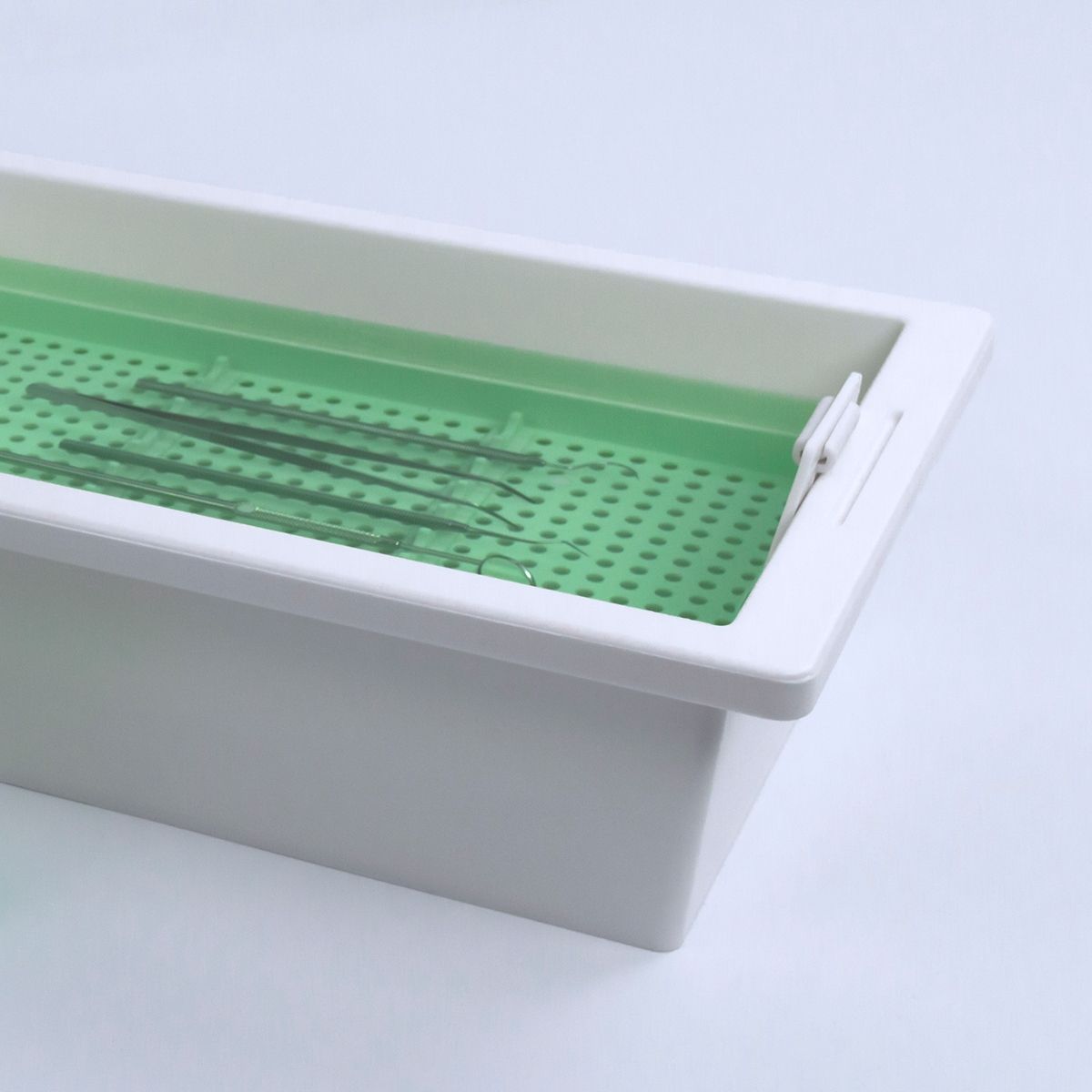
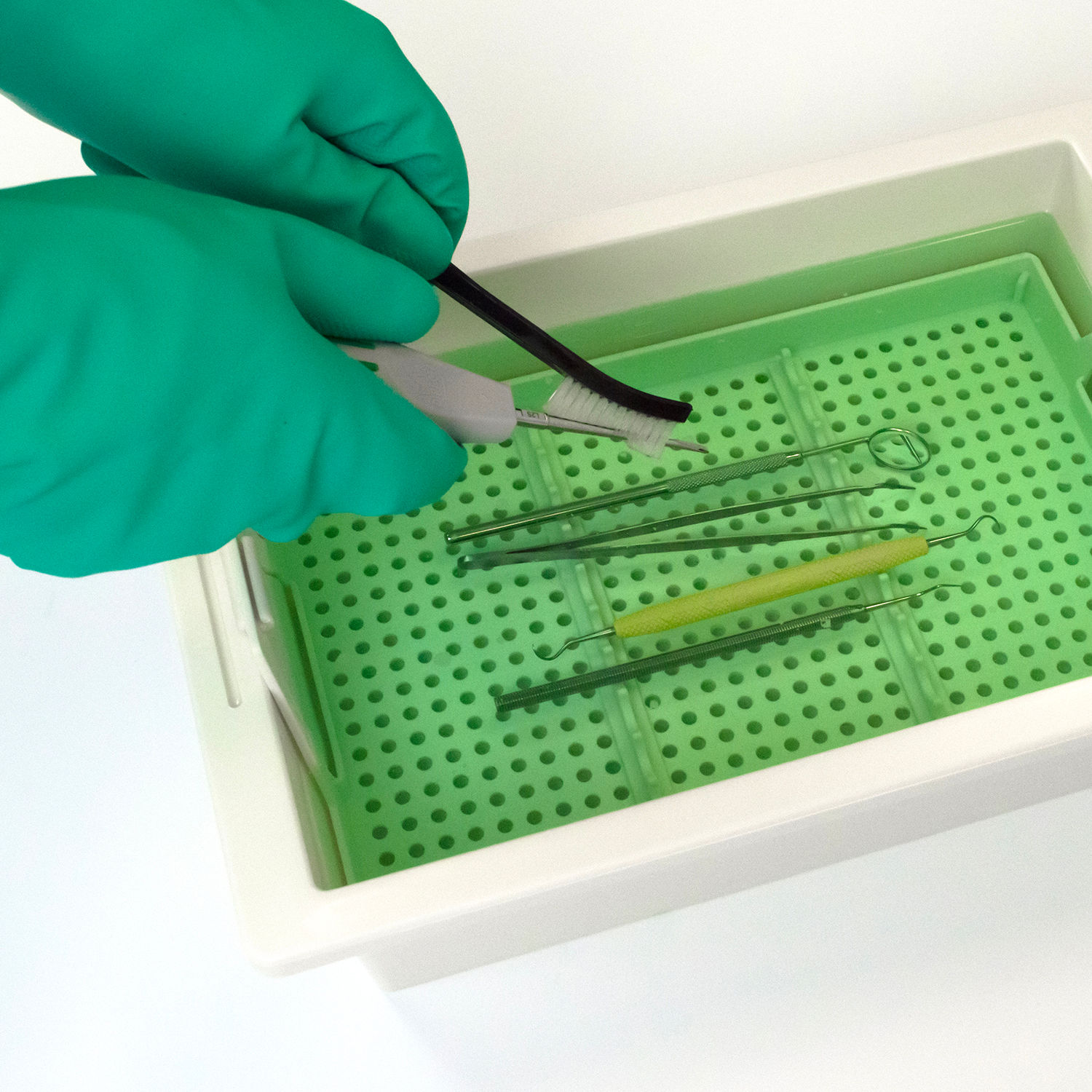
Bossklein IDactiv is a registered medical device, designed for the disinfection and cleaning of manual instruments and tools. Alternatively, IDactiv can also be used to disinfect many types of rotating instruments such as burs, diamonds and polishers inside the bur bath.
• For the disinfection of instruments and tools
• Use inside instrument bath or ultrasonic bath
• Short soaking time, from only 15 mins
• Super effective concentrate
• Wide material compatibility
• Low hazard classification
• Mild fresh aroma
• Virus tested
• Active against TB
• 2% or 4% dilution ratio
• 2 in 1: cleans & disinfects
Product Variants
| SKU | Variant Name |
|---|---|
| BOS9842 | 1L Dosing Bottle |
| BOS9843 | 2.5L Bottle With Dosing Cap |
| BOS9840 | 5L Bottle |
Size / Quantity:
Downloadable Information
Instructions For Use Safety Data Sheet (EN) Product Information Sheet Multi Language IFUProduct Information
- Test Standards
- Effective Spectrum & Exposure Times
- Product Composition
- Packing Details
- Physical Properties
- Material Compatability
- Directions For Use
- Additional Downloads
- HTM 01-05 Compliance
- Videos
- Hazards, CLP & Disposal
Test Standards
EN17111, EN14885, EN14561, EN14562, EN14563, EN14476, EN14348, EN13727, EN13624.Effective Spectrum & Exposure Times
| Micro-Organism | Contact Time | Concentration |
|---|---|---|
| BACTERICIDAL | 15 min | 2% |
| Pseudomonas aeruginosa | 15 min | 2% |
| Staphyloccocus aureus | 15 min | 2% |
| Enterococcus hirae | 15 min | 2% |
| E. coli | 15 min | 2% |
| Salmonella typhimurium | 15 min | 2% |
| MRSA | 15 min | 2% |
| Legionella pneumophila | 15 min | 2% |
| YEASTICIDAL | 15 min | 2% |
| Candida albicans | 15 min | 2% |
| ENVELOPED VIRUSES | 15 min | 2% |
| Vaccinia virus | 15 min | 2% |
| Hepatitis B Virus (HBV) | 15 min | 2% |
| Hepatitis C Virus (HCV) | 15 min | 2% |
| HIV | 15 min | 2% |
| Measles virus | 15 min | 2% |
| Hepatitis Delta Virus (HDV) | 15 min | 2% |
| Influenza virus | 15 min | 2% |
| Coronavirus | 15 min | 2% |
| Herpesviridae | 15 min | 2% |
| Filoviridae (Ebola) | 15 min | 2% |
| Rubella virus | 15 min | 2% |
| Rabies virus | 15 min | 2% |
| NON-ENVELOPED VIRUSES | ||
| Adenovirus 5 | 30 min | 4% |
| Norovirus | 30 min | 4% |
| MYCOBACTERICIDAL | 60 min | 4% |
| Mycobacterium avium | 60 min | 4% |
| TUBERCULOCIDAL | 60 min | 4% |
| Mycobacterium terrae | 60 min | 4% |
Product Composition
Aqua, N-(3-Aminopropyl)-N-Dodecylpropane-1,3-Diamine (4.1% w/w 2372-82-9), DMPAP (3.5% w/w), Alcohols, C9-11, Ethoxylated, Tetrasodium N,N-Bis(Carboxylatomethyl)-L-Glutamate, Monoethanolamine, Corrosion Inhibitor, Perfume contains CINEOLE - Cineols - Eucolytus; HYDRATROPALDEHYDE; TRANS-MENTHONE; p-menth-1-en-4-ol., Dye.Packing Details
6 x 1L Bottles per box, 4 x 2.5L Bottles per box, 2 x 5L UN Approved Refill Bottles per boxPhysical Properties
Green liquid, Mild Fresh aroma, pH 9-10, Shelf life of 3 yearsMaterial Compatability
EN21530 tested: Compatible with Stainless Steel 304, Stainless Steel 316, Stainless Steel 410, Stainless Steel 420, Stainless Steel 440, Acrylic glass, Carbon Steel, HDPE, Silicone rubber, Aluminium*, Titanium. *Do not immerse for longer than 24 hoursDirections For Use
Add 20ml ± 1ml to 980ml of clean water for a 2% solution, 40ml ± 1ml to 960ml for a 4% solution. To achieve full efficacy use a 4% solution for a 60 minute soaking time. After disinfection, rinse instruments thoroughly with RO or distilled water prior to further processing and sterilisation. Follow the reprocessing instructions from the instrument manufacturer. Change the solution daily. For professional use only.
Additional Downloads
Material Safety Data Sheets are available in a selection of different languages. Use the links below to view/download:
- Bossklein IDactiv MSDS – DE
- Bossklein IDactiv MSDS – ES
- Bossklein IDactiv MSDS – FR
- Bossklein IDactiv MSDS – NL
- Bossklein IDactiv MSDS – PT
- Bossklein IDactiv MSDS – RO
- Bossklein IDactiv MSDS – SV
HTM 01-05 Compliance
3.3: Manual cleaning, governed by an appropriate protocol, is acceptable within the essential-quality-requirements framework. Within the best-practice framework, however, manual cleaning should be considered only where the manufacturer specifies that the device is not compatible with automated processes (including ultrasonic cleaning) or when the washer-disinfector is temporarily unavailable (for example for repair or validation). Exceptionally, where local experience indicates that pre-washing may be helpful (for example in the removal of tenacious dental materials), such action may be appropriate before automated cleaning.
3.4: New instruments should be cleaned and sterilized before using for the first time, unless supplied as sterile.
3.42: Effective cleaning of dental instruments before sterilization is of the utmost importance to reduce the risk of transmission of infectious agents.
3.5: Instruments cleaned as soon as possible after use may be more easily cleaned than those left for a number of hours before reprocessing.
3.6 When working with substances that can harden on instruments (for example cements), the instruments should be cleaned immediately. Instruments that cannot be cleaned should be discarded.
3.9: Manual cleaning should be considered where manufacturers’ instructions specify that the device is not compatible with automated processes.
Videos
Hazards, CLP & Disposal
Danger. Causes skin irritation. Causes serious eye damage. Very toxic to aquatic life with long lasting effects. Wash hands thoroughly after handling. Wear protective gloves, eye protection, protective clothing. IF ON SKIN: Wash with plenty of water. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a doctor. If skin irritation occurs: Get medical advice/attention. Collect spillage.
Storage. Store in a well-ventilated place. Keep container tightly closed. Keep cool. Contraindications. Heat Disposal. Do not discharge concentrate into drains or rivers.
UFI: 3X00-W0GP-900K-VWW7
UN: 1903, Packing group: III, Class: 8, Shipping name: DISINFECTANT, LIQUID, CORROSIVE, N.O.S. (N-(3-AMINOPROPYL)-N-DODECYLPROPANE-1,3-DIAMINE)
Scope Of Application
Veterinary
Professional Care
Dental
Opticians
Beauty and Wellbeing
Medical and Chiropody
Download our brochure
Relevant product information from packaging info, effective spectrums, instructions for use, hazard classifications and much more.