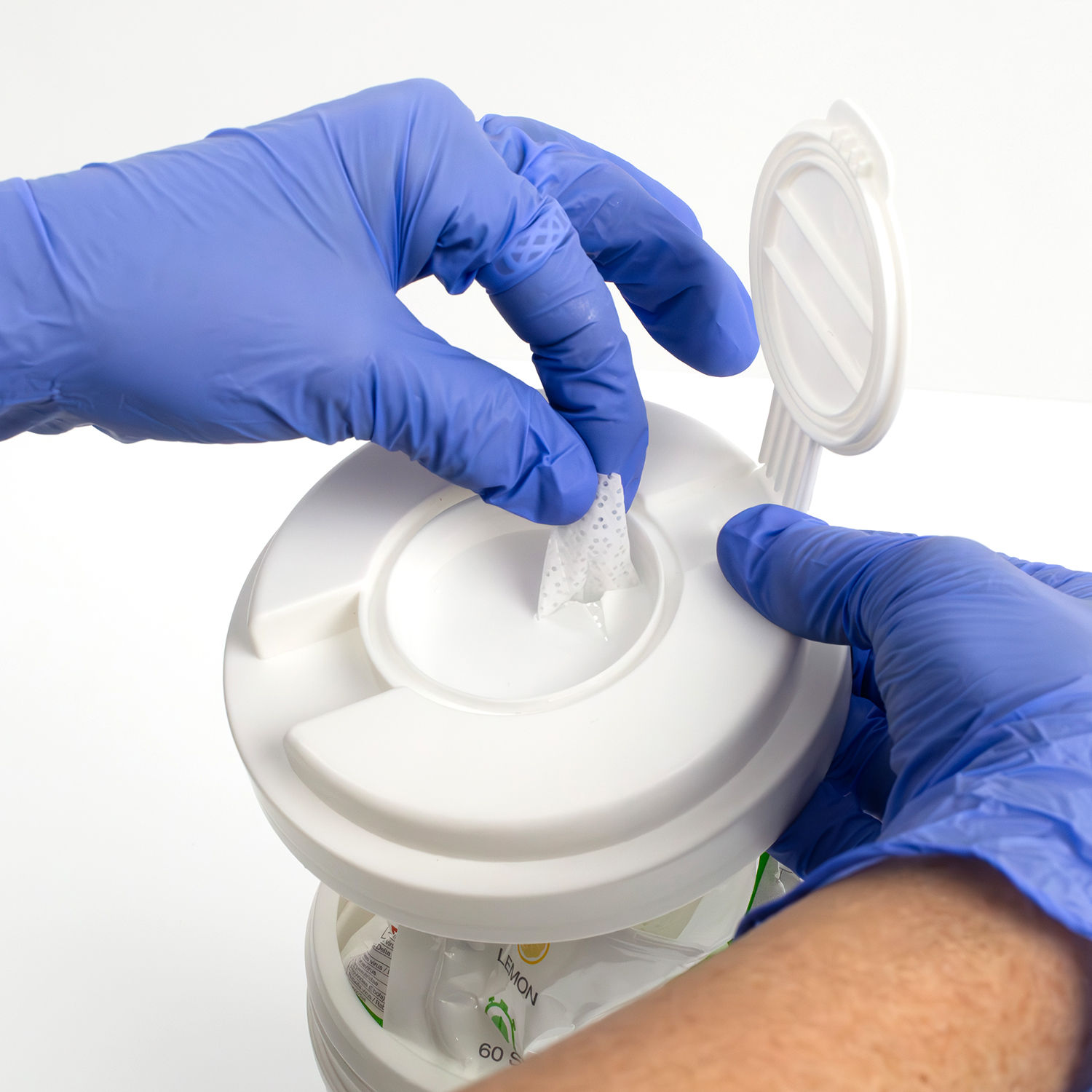
V-WIPE Ultra Wipes
Product Highlights
Large size surface disinfectant wipes, designed for use in the medical and dental environment for the surface disinfection of non-invasive medical devices.
• Packs contain 160 premium wipes, size 175x155mm
• High quality 40gsm wipe material
• Extra strong, extra durable
• For wipeable non-porous surfaces
• Alcohol based formulation 63% Ethanol w/w (70.5% v/v)
• Fresh lemon aroma
• EN14476 virus tested
• 60 second contact time
• Wide effective spectrum
Bossklein V-Wipe Surface Disinfectant Wipes are ideally suited for alcohol resistant materials and surfaces within the clinical environment.
Dual registration – approved for use on non invasive medical devices and also general surfaces under Biocidal Products Regulation (BPR)
The short contact time makes them ideal to use in between patients where time is crucial and the unique quick drying formula leaves surfaces decontaminated and streak free
Product Variants
| SKU | Variant Name |
|---|---|
| BOS1000 | Dispenser Tub of 160 wipes |
| BOS1001 | Economic Refill of 160 wipes |
| BOS1002 | 1 x Empty Tub + 6 x Refills of 160 wipes |
Size / Quantity: 175x155mm x 160 wipes
Downloadable Information
Instructions For Use Safety Data Sheet (EN) Product Information Sheet Multi Language IFU Compare all the V-WIPEsProduct Information
- Test Standards
- Effective Spectrum & Exposure Times
- Product Composition
- Packing Details
- Physical Properties
- Directions For Use
- Additional Downloads
- HTM 01-05 Compliance
- Hazards, CLP & Disposal
Test Standards
EN16615, EN14476, EN14348, EN13727, EN13624.Effective Spectrum & Exposure Times
| Micro-Organism | Contact Time |
|---|---|
| Bactericidal | 60 sec |
| Pseudomonas aeruginosa | 60 sec |
| Enterococcus hirae | 60 sec |
| Staphylococcus aureus | 60 sec |
| MRSA | 60 sec |
| VRE | 60 sec |
| E-Coli | 60 sec |
| Yeasticidal | 60 sec |
| Candida albicans | 60 sec |
| Enveloped Viruses | 60 sec |
| Hepatitis B Virus (HBV) | 60 sec |
| Hepatitis C Virus (HCV) | 60 sec |
| Human Immunodeficiency Virus (HIV) | 60 sec |
| RSV | 60 sec |
| Influenza Virus | 60 sec |
| Hepatitis Delta Virus (HDV) | 60 sec |
| Coronavirus | 60 sec |
| Filoviridae (which includes Ebola Virus) | 60 sec |
| Rubella Virus | 60 sec |
| Measles Virus | 60 sec |
| Herpesviridae | 60 sec |
| Rabies Virus | 60 sec |
| Poxviridae | 60 sec |
| Non Enveloped Viruses | |
| Adenovirus | 60 sec |
| Norovirus | 60 sec |
| Mycobactericidal | 60 sec |
| Mycobacterium avium | 60 sec |
| Tuberculocidal | 60 sec |
| Mycobacterium terrae | 60 sec |
Product Composition
Ethanol (64-17-5) 63% w/w (630g/kg), Aqua, Perfumes (Contains Linalool, Citral, Geraniol, Alpha-Isomethyl ionone).Packing Details
12 x Dispenser Tub of 200 wipes per box, 12 x Refill of 200 wipes per box, 6 x Refills of 200 wipes + 1 Dispenser Tub per box,Physical Properties
White substrate impregnated with clear liquid. Fresh lemon aroma. pH of solution 6.50-8.50, Shelf life of 3 years.Directions For Use
Place refill bag in dispenser tub. Feed the centre wipe through the hole in the cap and replace. Using sufficient wipes for each application wipe the clean surfaces to be disinfected so that they are completely moistened and let the disinfectant take action over 60 seconds. Allow surface moisture to evaporate. For professional use only.
Additional Downloads
Material Safety Data Sheets are available in a selection of different languages. Use the links below to view/download:
HTM 01-05 Compliance
5.3: The decontamination area should be wiped down carefully after each decontamination cycle is completed; for clinical areas, a similar wipe-clean is required after each patient procedure and before the next patient is admitted.
6.37: Surfaces and equipment used in the decontamination of dental instruments should be cleaned carefully before and after each decontamination process cycle. The procedure used should comply with written local policies.
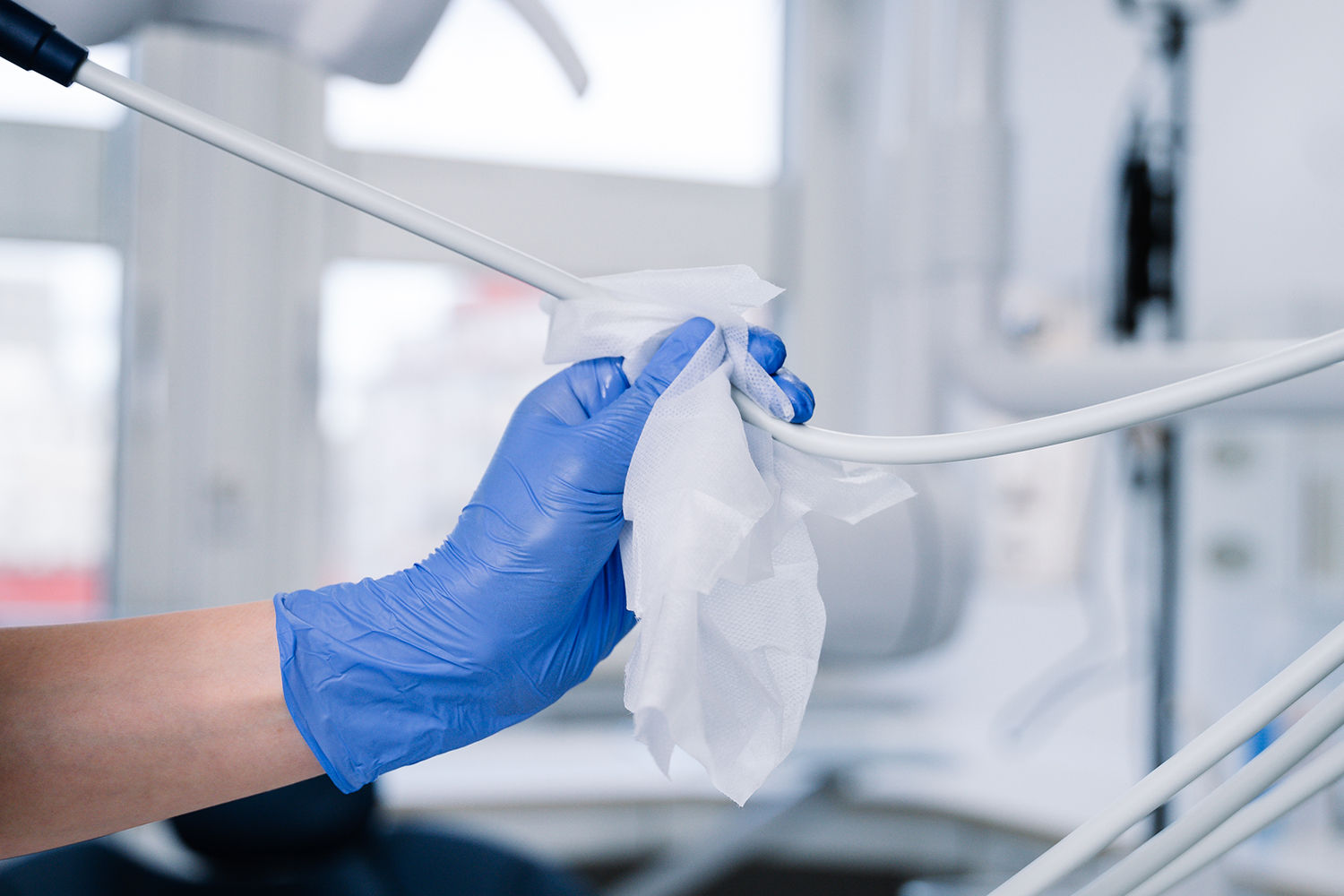
6.61: The patient treatment area should be cleaned after every session using disposable cloths or clean microfibre materials – even if the area appears uncontaminated.
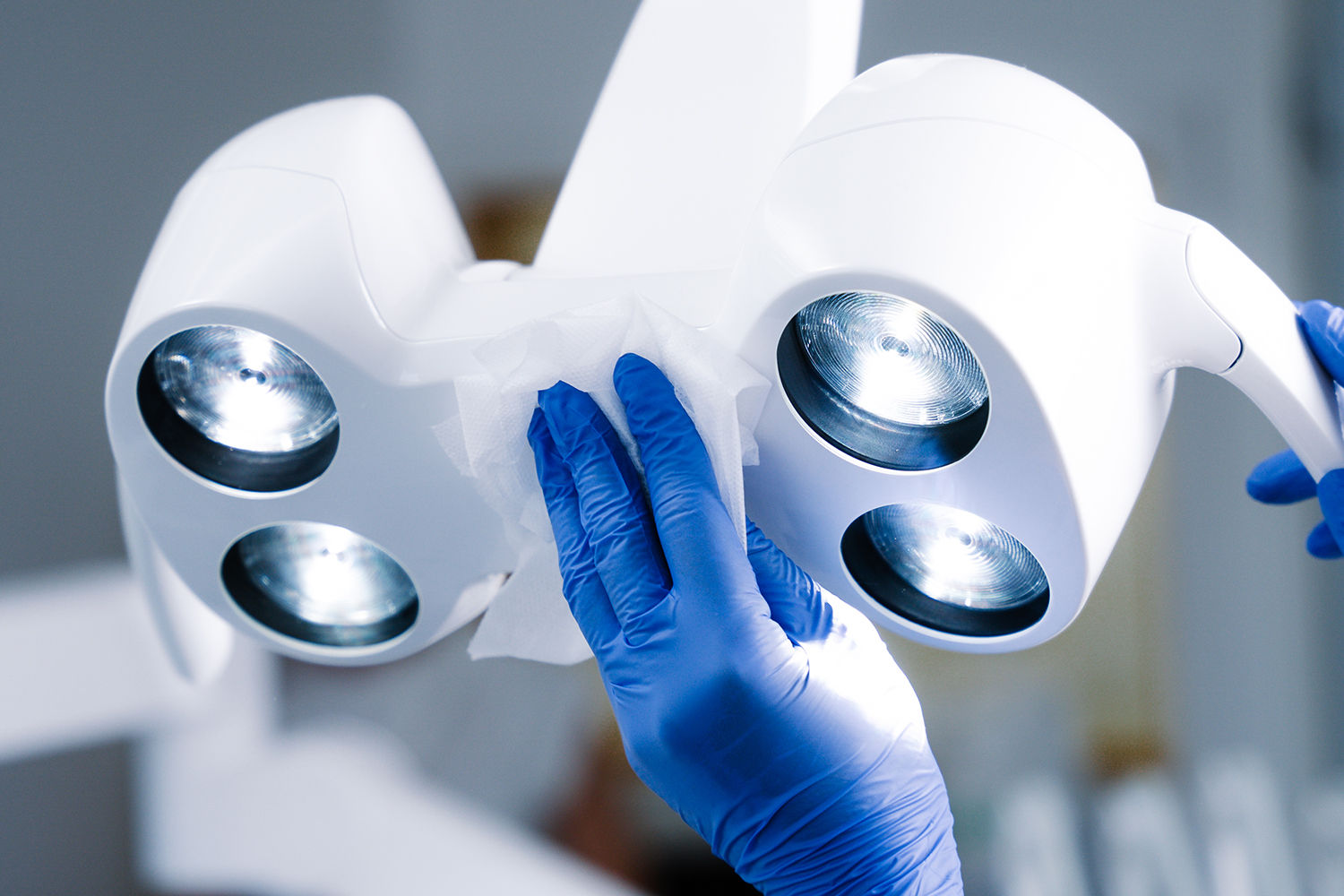
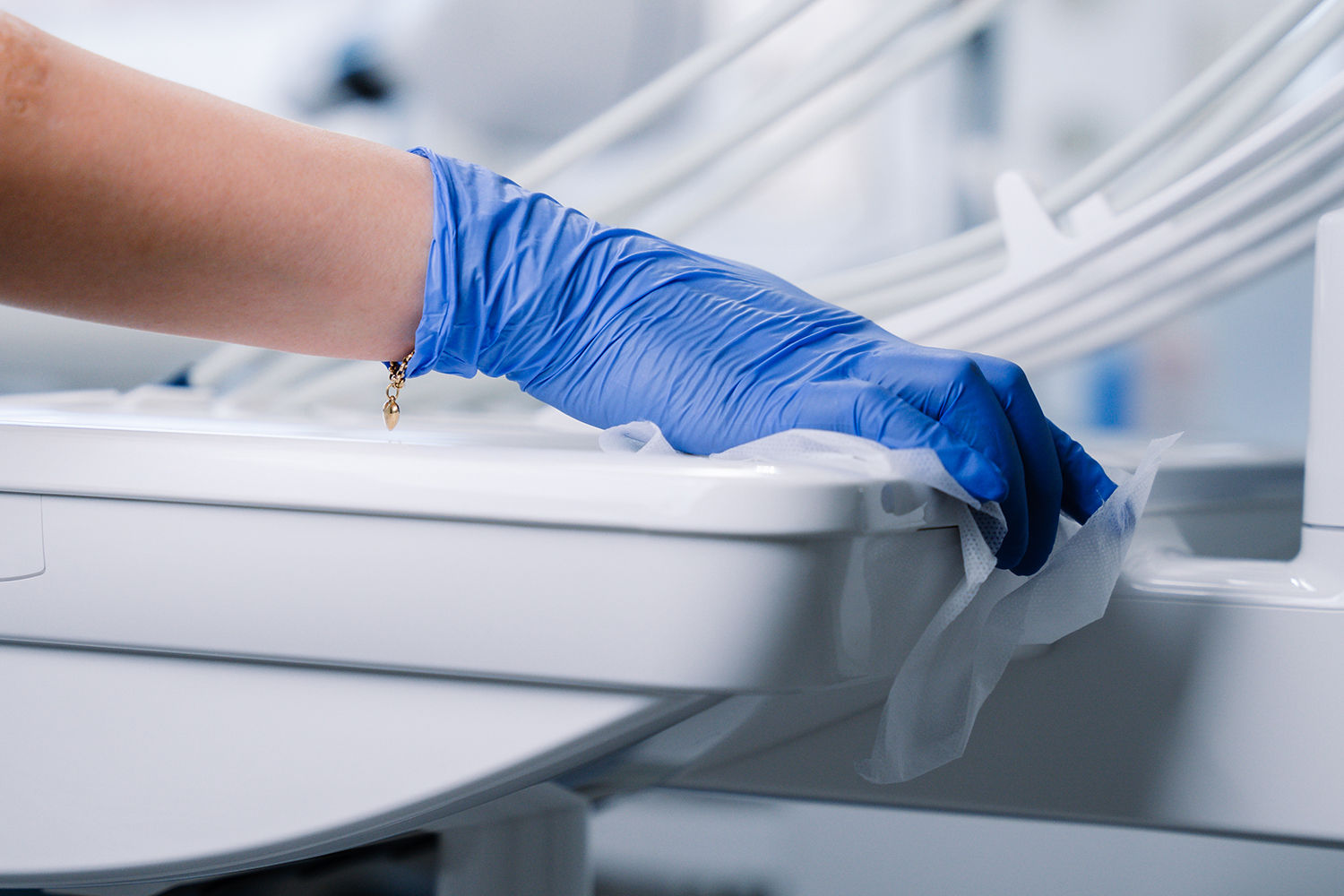
6.62: Areas and items of equipment local to the dental chair that need to be cleaned between each patient include:
- local work surfaces;
- curing lamps;
- inspection lights and handles;
- hand controls including replacement of covers;
- trolleys/delivery units;
- X-ray units.
Hazards, CLP & Disposal
Contraindications. Do not use on surfaces sensitive to alcohol. Disposal. Dispose of contents/container in accordance with local regulations. Do not discharge into drains or rivers. Do not flush. Non maceratable. UK number for poison advice: 111. Poisons Information Ireland: For information or to report a poisoning incident contact The National Poisons Information Centre (01 8092166).
Danger. H225: Highly flammable liquid and vapour. H319: Causes serious eye irritation. P280: Wear eye protection, protective gloves. P337+P313: If eye irritation persists: Get medical advice/attention. P370+P378: In case of fire: Use alcohol resistant foam to extinguish. Storage. P210: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P403+P233: Store in a well-ventilated place. Keep container tightly closed. P235: Keep cool.
UN: 3175, Packing group: ll, Class: 4.1, Shipping name: SOLIDS CONTAINING FLAMMABLE LIQUID, N.O.S. (ETHANOL)
UFI: EE00-V0C9-F00M-WVDU
FR 66132 N-114312 BE-REG-01369 2022-02-09-B05a 06/22/L bio/4180/D/24/CCHLP PCS 102769
Scope Of Application
Veterinary
Professional Care
Pharma
Food Production
Leisure
Institutions
Travel
Dental
Opticians
Hospitality
Beauty and Wellbeing
Medical and Chiropody
Download our brochure
Relevant product information from packaging info, effective spectrums, instructions for use, hazard classifications and much more.